Time is Limited - Act Now
Time is Limited
Act Now
100% Free & Secure Case Evaluations
Our personal injury attorneys are successful in fighting for the rights of consumers!
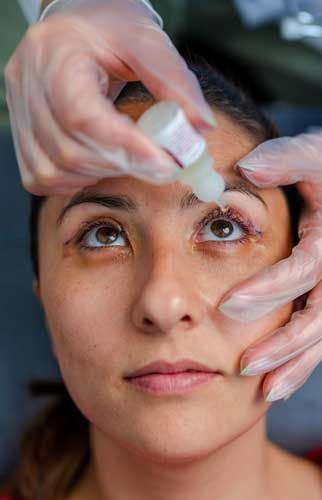
Victims of EzriCare Artificial Tears®, Delsam Pharmas Artificial Tears®, Delsam Pharmas Artificial Eye Ointment® could be eligible for justice, compensation.
Please seek the advice of a medical professional before making health care decisions. This advertisement is not associated with any government agency.
www.nationalcaseinfo.com is the property of Shield Legal LLC. 5170 Badura Ave Las Vegas, NV 89118
This website is not part of the Facebook website or Facebook, Inc. Additionally, this site is NOT endorsed by Facebook in any way. FACEBOOK is a trademark of FACEBOOK, INC.
ATTORNEY ADVERTISING. This Website is not intended to provide medical advice. Consult your doctor or physician before starting or stopping any medication.
Discontinuing a prescribed medication without your doctor’s advice can result in injury or death. are not an indication of future results. Every case is evaluated on its own facts and circumstances. Valuation depends on facts, injuries, jurisdiction, venue, witnesses, parties, and testimony, among other factors. No representation is made that the quality of legal services to be performed is greater than the quality of legal services performed by other lawyers. National Case Info does not itself provide legal services. Cases will be referred to third party attorneys and law firms. Do not rely on this advertisement in making any medical decision. Please call your physician before making any medical decision, including altering your use of any drug. Court costs and case expenses may be the responsibility of the client. Not available in all states. This advertisement is not intended as a testimonial, endorsement or dramatization, and does not constitute a guarantee, warranty, or prediction regarding the outcome of your legal matter, either expressed or implied. Anyone considering a lawyer should independently investigate the lawyers' credentials and ability, and not rely upon advertisements or self-proclaimed expertise. Only persons age 18 or older have permission to access our Service. Our Service does not address anyone under the age of 13("Children").
Privacy Policy | Terms and Conditions | CCPA Privacy Notice | Do Not Sell My Info
© 2025 National Case Info. All Rights Reserved